Direction of Dipoles is represented by an arrow toward negative poles and a cross at the positive pole. the positive dipole is indicated as follows
-I-->
H Cl
the negative region in one polar molecule attracts the positive region in adjacent molecules. the moleules are attracted to each other by opposite sides.
each forces of attraction between polar moleules are known as a dipole- dipole forces
dipole-dipole foreces act at short range only between nearyby molecules.
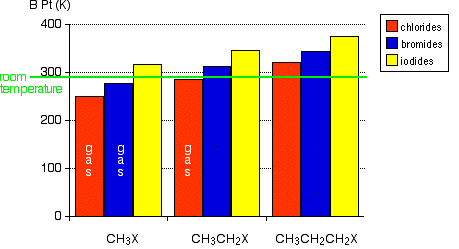
this explains the high boiling point in molcules with dipole dipole forcesex I-Cl (97 c) but Br-Br (59 c)
a polar molecule can induce a dipole in a non polar molecule by temporarily attracting its electrons
the result is a short range intermolecular force that is weaker than a dipole-dipole force.
this accounts for the oxygen's ability to be dissolved into water
some hydrogen containg compounds have high boiling points. explored by a particularly strong of dipole-dipole force
examples are phosphorous and sulfur
this gives a hydrogen atom a positive charge that is almost half that of a bare proton
the small size of the hydrogen atom allows it come close to an unshared pair in another atom
this forms a hydrogen bond
these are connected with a dotted line
excellent example is water
properties aquired from hydrogen bonding are surface tension, cohesion, solvent, and boiling point is raised
london dispersion theory
even noble gas atoms and non polar molecules can experience weak intermolceular bonding
in any atom or molecule the electrons are in constant motion
thus there exists a possibilty of there being a more positive and negative side which attracts the more negative or positive sides of a different atom or molecule
this is the weakest type of bond
suggested in 1930


